1. CHEMICAL PRODUCTS & COMPANY IDENTIFICATION
Product Name: Uranium Hexafluoride
Formula: UF6
CAS RN: [7783-81-5]
Chemical Family: Inorganic salt, radioactive
Supplier
IBI Labs
3495 N. Dixie Hwy. Unit # 8
Boca Raton, FL 33431
Tel: 561-826-0061 Fax: 561-892-8450
Emergency Telephone Numbers
INFOTRAC
USA & Canada contact number: 1-800-535-5053
International contact number: 1-352-323-3500
2. HAZARDS IDENTIFICATION
OSHA Hazards
Highly toxic by inhalation, highly toxic by ingestion, corrosive.
Target Organs: Kidney, liver, lungs, brain, skin, eyes.
GHS Classification
Acute toxicity, Oral (Category 1)
Acute toxicity, Inhalation (Category 1)
Specific target organ toxicity – repeated exposure (Category 2)
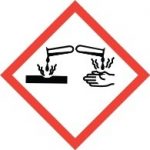
Skin Corrosion (Category 1A)
Serious eye damage (Category 1)
Acute aquatic toxicity (Category 2)
Chronic aquatic toxicity (Category 2) GHS Label elements, including precautionary statements.
Pictogram
Signal Word: Danger
Hazard Statements
H300 + H330 Fatal if swallowed or if inhaled.
H314 Causes severe skin burns and eye damage.
H373 May cause damage to organs through prolonged or repeated exposure.
H411 Toxic to aquatic life with long lasting effects.
Precautionary Statements
P260 Do not breathe dust/ fume/ gas/ mist/ vapors/ spray.
P262 Do not get in eyes, on skin, or on clothing.
P264 Wash skin thoroughly after handling.
P273 Avoid release to the environment.
P280 Wear protective gloves/protective clothing/eye protection/face protection.
P310 Immediately call a POISON CENTER or doctor/ physician if swallowed or inhaled.
Other Hazards: Radioactive
CERCLA Ratings (SCALE 0-3): HEALTH=3 FIRE=0 REACTIVITY=1 PERSISTENCE = 3
NFPA RATINGS (SCALE 0-4); HEALTH=4 FIRE=0 REACTIVITY=1
Emergency Overview
- Volatile.
- Colorless or white, deliquescent monoclinic crystal solid.
- It may be fatal if inhaled.
- It may cause blood disorders, eye damage, burns, and convulsions.
- It may damage the kidneys.
- It may affect the central nervous system, liver, and breathing.
- It may cause adverse reproductive effects.
- It may react with water.
Poison:
- Do not breathe fumes.
- Do not get in the eyes, on the skin, or clothing.
- Do not allow water to get in the container.
- Keep the container tightly closed.
- Wash thoroughly after handling.
- Use only with adequate ventilation.
- Handle with caution.
3. HEALTH EFFECTS
Short-Term Effects:
- Inhalation: This may cause a lack of appetite, nausea, vomiting, diarrhea, dehydration, kidney damage, blood in the urine, jaundice, weakness, drowsiness, incoordination, twitching, sterility, blood disorders, convulsions, and shock. Exposure to radioactive substances increases one’s risk of developing cancer.
- Skin: This may cause skin irritation and kidney damage.
- Eyes: May irritate. Additionally, eye damage, including ulcerations, may occur.
- Ingestion: This may cause drooling, nausea, vomiting, diarrhea, stomach pain, weakness, twitching, kidney damage, and convulsions. Also, may cause increased cancer risk.
Long-Term Effects:
- Inhalation: In addition to effects from short-term exposure, anemia, cataracts, and lung damage may occur.
- Skin: In addition to effects from short-term exposure, liver damage may occur.
- Eyes: It is unlikely that long-term eye contact would occur as the effects of short-term exposure, over a period, would result in serious eye damage. However, if long-term exposure did occur, cataracts may also occur.
- Ingestion: No information is available on significant adverse effects. Prolonged exposure to uranium compounds may cause kidney damage and elevated cancer risk.
Carcinogen Status
OSHA: N
NTP: N
IARC: N
4. COMPOSITION AND INFORMATION ON INGREDIENTS, PHYSICAL AND CHEMICAL PROPERTIES
- Component: Uranium Hexafluoride
- Percentage: 100.0
- Other Contaminants: None
- Appearance: Volatile, colorless, or white, deliquescent monoclinic crystal solid.
- Molecular Weight: 352.019
- Molecular Formula: UF6
- Boiling Point: 56°C
- Melting Point: 64°C
- Water Solubility: Reacts vigorously.
- Solvent Solubility: Fluorocarbons, liquid chlorine and bromine, nitrobenzene, carbon tetrachloride, chloroform, tetrachloroethane
- Odor: Pungent
- Odor Threshold: .04ppm
Half-Life
The half-lives of the various uranium isotopes are as follows:
234U = 2.47 X 105 y; 235U = 7.04 X 108 y; 236U = 2.39 X 107 ; 238U = 4.51 X 109 y; 233U = 1.59 X 105 y.
Specific Activity
The specific activities of the various uranium isotopes are as follows:
233U = 3.6 X 102 MBq/g (9.7 X 10-3 Ci/g)
234U = 2.3 X 102 MBq/g (6.2 X 10-3 Ci/g)
235U = 7.8 X 10-2 MBq/g (2.1 X 10-6 Ci/g)
236U = 2.3 MBq/g (6.3 X 10-5 Ci/g)
238U = 1.2 X 10-2 MBq/g (3.3 X 10-7 Ci/g)
5. FIRST AID MEASURES
- UF6 upon exposure to air will generate hydrogen fluoride and uranyl fluoride.
- Hydrofluoric acid burns require immediate and specialized first aid and medical treatment.
- Symptoms may be delayed for up to 24 hours.
- Skin exposures can be treated with calcium gluconate.
- Conditions such as hypocalcemia, hypomagnesemia, and cardiac arrhythmias should be monitored.
Inhalation:
- Remove from the exposure area to a restricted area with fresh air as quickly as possible.
- If breathing has stopped, perform artificial respiration, preferably by administering oxygen.
- Get medical attention immediately.
- Medical problems take priority over radiological concerns.
- The victim may be contaminated with radioactive particles.
Skin contact:
- Remove the victim to a suitable area for decontamination as quickly as possible.
- Remove clothing and shoes immediately.
- Thoroughly wash the victim with soap and water.
- Medical problems take priority over radiological concerns.
- Upon completion of washing, monitor the victim for radioactivity.
Eye contact:
- Remove the victim to a restricted area for decontamination.
- Thoroughly wash eyes with water, occasionally lifting the upper and lower lids (approximately 15 minutes).
- Following the water treatment, provide an isotonic solution.
- Monitor the victim for radioactivity. If activity is present, rewash the eyes, and re-monitor until little or no radioactivity is present.
- Medical problems take priority over radiological concerns.
- Get medical attention immediately.
Ingestion:
- In the case of the ingestion of radioactive substances, the mouth should be rinsed out immediately after the accident.
- Medical problems take priority over radiological concerns.
- Get medical attention immediately.
6. FIREFIGHTING MEASURES
Fire and Explosion Hazards
- Negligible fire hazard when exposed to heat or flame.
- Slight explosion hazard when exposed to heat or flame.
Extinguishing Media: Dry chemical or carbon dioxide. (Emergency Response Guidebook (ERG), developed jointly by Transport Canada (TC), the U. S. Department of Transportation (DOT), and the Secretariat of Transportation and Communications of Mexico (SCT).)
For larger fires, use water spray, fog, or regular foam (Emergency Response Guidebook, ERG.)
Firefighting
- Move the container from the fire area if you can do it without risk.
- Apply cooling water to the sides of containers exposed to flames until well after the fire is out.
- Stay away from the ends of tanks.
- If this is impossible, withdraw from the area and let it fire (Emergency Response Guidebook, ERG).
- Contact the local, state, or Department of Energy radiological response team.
- Do not allow water to get on spilled material.
- Use water in flooding quantities as a fog if combustible materials are involved.
- Knockdown vapors with water spray.
- Cool fire exposed containers with flooding quantities of water applied from as far a distance as possible.
- Avoid breathing dust and fumes; keep upwind.
Hazardous Combustion Products
Thermal decomposition products may include corrosive fumes of hydrogen fluoride, and toxic, and hazardous fumes of uranium.
7. ACCIDENTAL RELEASE MEASURES
Occupational spill:
- Do not touch spilled material.
- Try to freeze leakage by cooling at the point of opening with carbon dioxide (dry ice).
- Use water spray to reduce vapor; do not put water directly on lead or spill area.
- For small spills, flush the area with flooding amounts of water on a small part of the spill at a time.
- Keep unnecessary people away; isolate hazard areas and deny entry.
- Stay upwind; keep out of low areas.
- Delay cleanup until arrival or instruction of qualified radiation authority.
8. HANDLING AND STORAGE
Observe all Federal, State, and local regulations when storing this substance.
9. EXPOSURE CONTROLS AND PERSONAL PROTECTION
Exposure Limits
Uranium, soluble compounds (As U):
0.05 mg/m3 OSHA PEL TWA
0.2 mg/m3 ACGIH TLV TWA; 0.6 mg/m3 ACGIH STEL
0.05 mg/m3 NIOSH Recommended TWA
Hydrogen Fluoride
6 ppm OSHA STEL (15 minutes), 3.0 ppm OSHA PEL
0.5 ppm ACGIH TLV TWA, 2 ppm ACGIH Ceiling
6 ppm NIOSH Ceiling, 3 ppm NIOSH REL TWA (10 hours)
Occupational exposure to radioactive substances must adhere to standards established by the Occupational Safety and Health Administration, 29 CFR 1910.96, and/or the Nuclear Regulatory Commission, 10 CFR Part 20.
Ventilation
- At a minimum, provide local exhaust or process enclosure ventilation.
- Depending upon the specific workplace activity and the radioactivity of the isotope, a more stringent ventilation system may be necessary to comply with exposure limits set forth by law (10 CFR 20.103).
- Radioactive exposure levels should be maintained As Low As Reasonably Achievable (ALARA).
Shielding
Alpha particles: For the energy range of alpha particles usually encountered, a fraction of a millimeter of any ordinary material or a few inches of air is sufficient for absorbency.
Beta particles: Beta particles are more penetrating than alpha and require more shielding. Materials composed mostly of elements of low atomic number such as acrylic, and thick rubber are most appropriate for the absorption of beta particles. Uranium hexafluoride, in quantities used for Certified Reference Materials and SME materials, does not emit significant amounts of beta particles.
Gamma rays: The most suitable materials shielding gamma radiation are lead and iron. Uranium, in quantities used for Certified Reference Materials and SME materials, does not emit significant amounts of gamma radiation. Consult a radiation protection specialist or health physicist for more information.
Alpha-Neutron reaction: Neutrons of approximately 2 MeV are generated by the interaction of alpha particles from uranium with the nuclei of fluoride and other low-Z atoms. The magnitude of the neutron flux will vary based on the total activity of uranium (which is a function of enrichment) and the chemical compound in question (mixing of U and F). In the case of a storage cylinder of UF6, the typical neutron dose rate from natural to 5% enriched uranium would be expected to be 0.01 to 0.2 mrem/hr. Neutron dose rates from P-10 tubes would be even lower.
Eye protection: Employees must wear appropriate eye protection that will not allow the introduction of particles into the eyes. Contact lenses should not be worn.
Clothing: The laboratory uses only protective clothing is required. In the event of an accident, large-scale release or a large-scale clean-up of full protective clothing will be necessary.
Gloves: Employees must wear appropriate protective gloves to prevent contact with this substance. Used gloves should be disposed of as radioactive waste.
Respirator: The following respirators and maximum use concentrations are recommendations by the U.S. Department of Health and Human Services, NIOSH pocket guide to chemical hazards; NIOSH criteria documents or by the U.S. Department of Labor, 29 CFR 1910 Subpart Z.
The specific respirator selected must be based on contamination levels found in the workplace, must not exceed the working limits of the respirator, and be jointly approved by the National Institute for Occupational Safety and Health and the Mine Safety and Health Administration (NIOSH-MSHA).
URANIUM, SOLUBLE COMPOUNDS (As U), HALIDES
At any detectable concentration:
At any detectable concentration: Any self-contained breathing apparatus with a full facepiece operated in a pressure-demand or other positive-pressure mode.
Any supplied-air respirator with a full facepiece operated in a pressure-demand or other positive-pressure mode or other positive-pressure mode with an auxiliary self-contained breathing apparatus operated in pressure-demand or other positive-pressure mode.
Escape – any air-purifying, full-facepiece respirator with a high-efficiency particulate filler.
Any appropriate escape-type, self-contained breathing apparatus.
For firefighting and other immediately dangerous for life or health conditions:
Use any self-contained breathing apparatus with a full facepiece respirator and a high-efficiency particulate filter.
Use any supplied-air respirator with a full facepiece operated in a pressure-demand or other positive-pressure mode or other positive-pressure mode with an auxiliary self-contained breathing apparatus operated in pressure-demand or other positive-pressure modes.
10. STABILITY AND REACTIVITY
Reactivity
Uranium Hexafluoride: Vigorous, exothermic reaction with water releases corrosive hydrogen fluoride and toxic uranyl fluoride.
This compound will attack some plastics, rubbers, and coatings. These materials should not be used to contain uranium hexafluoride.
Conditions to avoid: May ignite combustible materials such as paper, wood, and oil. The container may explode in heat or fire.
Incompatibilities
Uranium Hexafluoride: Aromatic Hydrocarbons and Hydroxy compounds. Interaction with benzene, toluene, or xylene is very vigorous with separation of carbons.
Water: Vigorous, exothermic reaction releases corrosive hydrogen fluoride and toxic uranyl fluoride.
Metals: Reacts with most metals; should be handled in a copper apparatus.
Hazardous Decomposition
Thermal decomposition products may include corrosive fumes of hydrogen fluoride, and toxic hazardous fumes of uranium.
Polymerization
Hazardous polymerization has not been reported to occur under normal temperature and pressure.
11. TOXICOLOGY INFORMATION
Uranium hexafluoride, upon exposure to moisture in the air, will release hydrogen fluoride which is a poisonous and corrosive gas as well as uranyl oxyfluoride which is a nephrotoxin. The material usually has a low level of radioactivity with the chemical hazard greatly exceeding the radiation hazard for all but highly enriched materials. Hydrogen fluoride is highly corrosive to the skin, eyes, and respiratory tract. The primary hazard of inhalation of uranium hexafluoride (byproduct hydrogen fluoride) is its corrosivity.
Uranium hexafluoride (byproduct hydrogen fluoride) is also highly toxic by inhalation. Poisoning affects the kidneys, liver, lungs, and hematopoietic system and causes disorders in protein and carbohydrate metabolism. Uranium poisoning may be acute or chronic and associated with both chemical and radiological toxicity. Uranium is a weak alpha emitter. Cancer of the lung, osteosarcoma, and lymphoma have all been reported because of radiation exposure to uranium. Deaths reported from acute inhalation of uranium hexafluoride have been due to the corrosive effect of the compound on the lungs.
12. HEALTH EFFECTS
Inhalation
Uranium Hexafluoride: Radioactive/Nephrotoxin/Highly Toxic.
- Acute exposure: Serious cases of poisoning have been observed after workers were exposed to uranium hexafluoride. The kidney is the organ most directly affected by soluble uranium compounds, such as uranium hexafluoride. Effects on the kidney are more severe and occur before effects on the liver. The deleterious effects of uranium on the liver are a result of changes in blood chemistry brought on by renal dysfunction.
- Chronic exposure: Chronic symptoms may develop after prolonged contact with soluble uranium compounds. The degree of symptoms depends on the level and time of exposure. Chronic exposure frequently results in changes in the blood, these changes may be due in part to the radioactive properties of uranium. The toxic effects of uranium compounds may also result in kidney failure, chronic hepatitis, gastritis, and other conditions.
Alpha Radiation
- Acute exposure: Alpha radiation will kill cells immediately adjacent to the source of contact. Large insoluble particles may remain at or near the site of deposition and cause local damage. Soluble compounds may rapidly enter the bloodstream. The damage depends on how quickly they are eliminated, and the susceptibility of the tissue in which they are stored.
- Chronic exposure: The effects of chronic exposure by internally deposited alpha-active material are dependent upon the amount, enrichment, and tissue. If large amounts become internally deposited, lung cancer, sterility, anemia, leukemia, or bone cancer may occur.
Skin Contact
Uranium Hexafluoride: Radioactive/Nephrotoxin.
- Acute exposure: Contact with uranium hexafluoride can cause severe irritation to the skin. Uranium hexafluoride has been reported to be absorbed through intact human skin. The effects of skin contact may then result in kidney toxicity usually associated with inhalation or other routes.
- Chronic exposure: Repeated or prolonged contact may result in irritation, dermatitis, and kidney failure. Kidney dysfunction can cause liver problems and the symptoms of chronic hepatitis. Uranium tends to seek the kidney and bone. Over time, alpha emission may cause radiation damage to tissues in which uranium has been deposited.
Eye Contact
Uranium Hexafluoride: Radioactive.
- Acute exposure: Uranium hexafluoride was shown to cause moderately severe injury when dropped into the eyes of rabbits, guinea pigs, and mice. Contact lenses should not be worn when using this compound, or any soluble uranium compound.
- Chronic exposure: Repeated or prolonged eye contact may result in irritation and conjunctivitis, or symptoms of radiation injury such as cataracts. See the following sections for information about alpha radiation and the eyes, as well as radiation sickness.
Alpha Radiation
- Acute exposure: Repeated or prolonged exposure to alpha radiation may result in cataract formation. Of the well-documented late effects of radiation on man, leukemia, and cataracts have been observed at doses lower than those producing skin scarring and cancer or bone tumors. The lens of the eye is a critical organ for radiation exposure. It is important to note that long-term eye contact with uranium hexafluoride would most likely result in serious damage to the cornea before cataracts would be formed. Normal usage of Certified Reference Materials or SME materials will not result in significant eye exposures except in cases of accidents or poor laboratory practice.
- Chronic exposure: Repeated or prolonged exposure to alpha radiation may result in cataract formation. See acute exposure.
Ingestion
Uranium Hexafluoride: Radioactive/Nephrotoxin.
- Acute exposure: Oral toxicity of uranium compounds is lower than that by inhalation. Uranium compounds are toxic to the kidneys. Absorbed uranium is deposited in bone and the kidneys. Alpha emission from deposited uranium may damage either of these tissues. Poisoning may also occur from the fluoride component of this material. Fluoride poisoning may result in excessive salivation, nausea, vomiting, diarrhea, and abdominal cramps. Fluoride-binding bivalent cations (calcium and magnesium) may result in weakness, tremors, shallow respiration, and convulsions and eventually lead to cardiac arrhythmias.
- Chronic exposure: Chronic poisoning may develop after prolonged exposure to soluble uranium compounds. There may be changes in the peripheral blood, such as leucopenia, lymphopenia, and neutropenia, and symptoms of vegetative dystonia. In addition to these chemical effects, long-term irradiation of the kidney and bone marrow may damage these tissues.
Alpha Radiation
- Acute exposure: The fate of ingested alpha emitters depends on their solubility and valence.
- Chronic exposure: Repeated ingestion of alpha emitters may increase cancer risks.
13. ECOLOGICAL INFORMATION
Environmental Impact Rating (0-4): 2
Acute Aquatic Toxicity: Acute and chronic aquatic toxicity (Category 2)
14. DISPOSAL INFORMATION
Observe all federal, state, and local regulations when disposing of this substance.
15. TRANSPORTATION INFORMATION
The U.S. Department of Transportation (D.O.T.) Code of Federal Regulations (49 CFR Parts 100-185), the International Air Transportation Association (IATA), the International Civil Aviation Organization (ICAO), and the International Maritime Organization (IMDG) are all factored into the classification and transport of material.
Proper Shipping Name:
Hazard Class:
UN/ID Number: To be determined on a case-by-case basis.
Special Information:
Packing Group:
The classification of substances with multiple hazards must be determined following the criteria presented in the regulations mentioned above. Due to the various quantities and combinations of materials being shipped at one time, the information above must be determined based on the characteristics of the specific shipment.
16. REGULATORY INFORMATION
TSCA STATUS: N
CERCLA SECTION 103 (40 CFR 302.4): N
SARA SECTION 302 (40 CFR 355.30): N
SARA SECTION 304 (40 CFR 355.40): N
SARA SECTION 313 (40 CFR 372.65): N
OSHA PROCESS SAFETY (29 CFR 1910.119): N
CALIFORNIA PROPOSITION 65: N
SARA HAZARD CATEGORIES, SARA SECTIONS 311/312 (40 CFR 370.21)
ACUTE HAZARD: Y
CHRONIC HAZARD: Y
FIRE HAZARD: N
REACTIVITY HAZARD: Y
SUDDEN RELEASE HAZARD: N
17. OTHER INFORMATION
Copyright 1994 IBI Labs. License granted to make unlimited paper copies for internal use only.
IBI Labs requires that those who receive their materials comply with 29 CFR 1910.1200(h), which mandates that employers provide employees with effective information and training about hazardous chemicals in their workplace.
The contents of this document are believed to be accurate as of the date of revision and are provided in good faith. However, it is recommended that recipients use this information as supplementary and exercise caution and judgment regarding its accuracy and suitability. Please note that IBI Labs cannot accept responsibility for any indirect, incidental, or consequential damage resulting from the use of the information provided in this Safety Data Sheet.
IBI Labs makes no warranties, expressed or implied, including warranties of merchantability and fitness for a particular purpose. This information is provided without warranty, and any use of the product that does not conform to this Safety Data Sheet, or that is used in combination with any other product or process, is the responsibility of the user.
Revision Date: 04/22/2024