1. PRODUCT AND COMPANY IDENTIFIERS
Name: Ammonium Uranyl Carbonate
Synonyms: Tetraammonium uranyl tricarbonate, of uranium depleted in uranium 235, Uranate(4-), tris[carbonato(2-)-O,O’]dioxo-, tetraammonium, (HB-8-22-111’1’1”1”), Azanium uranium carbonate
Formula: (NH4)4(UO2)(CO3)3
Structure:
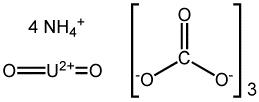
Uses: The substance is used in uranium yellow glazes.
Supplier
IBI Labs
3495 N. Dixie Hwy. Unit # 8
Boca Raton, FL 33431
Tel: 561-826-0061 Fax: 561-892-8450
Emergency Telephone Numbers
INFOTRAC
USA & Canada contact number: 1-800-535-5053
International contact number: 1-352-323-3500
2. REGISTRY NUMBERS AND INVENTORIES
CAS RN: [18077-77-5]
NIH PubChem CID: 3015006
EC (EINECS/ELINCS): 241-988-5
UN (DOT): 2909
Merck: 12,602
Beilstein/Gmelin: N/A
3. PHYSICAL AND CHEMICAL PROPERTIES
- Formula Mass: 522.21
- Molar Mass: 522.199 g/mol
- Melting Point: Decomposes between 165°C and 185°C
- Solubility: Insoluble in water
Ammonium uranyl carbonate (UO2CO3·2(NH4)2CO3) is known in the uranium processing industry as AUC and is also called uranyl ammonium carbonate. This compound is important as a component in the conversion process of uranium hexafluoride (UF6) to uranium dioxide (UO2). The ammonium uranyl carbonate is combined with steam and hydrogen at 500-600°C to yield UO2.
In another process aqueous uranyl nitrate, known as uranyl nitrate liquor (UNL) is treated with ammonium bicarbonate to form ammonium uranyl carbonate as a solid precipitate. This is separated from the solution, dried with methanol, and then calcinated with hydrogen directly to UO2 to obtain a sinterable grade powder. The ex-AUC uranium dioxide powder is free flowing, relatively coarse (10 µ), and porous with a specific surface area in the range of 5m2/g, and suitable for direct palletization, avoiding the granulation step. Conversion to UO2 is often performed as the first stage of nuclear fuel fabrication.
The AUC process is followed in South Korea and Argentina. In the AUC route, calcination, reduction, and stabilization are simultaneously carried out in a vertical fluidized bed reactor. In most countries, sinterable grade UO2 powder for nuclear fuel is obtained by the ammonium diuranate (ADU) process, which requires several more steps.
Ammonium uranyl carbonate is also one of the many forms called yellowcake in this case it is the product obtained by the heap leach process.
4. HAZARDS AND PROTECTION
Storage:
- Keep in a cool, dry, dark location in a tightly sealed container or cylinder.
- Keep away from incompatible materials, ignition sources, and untrained individuals.
- Secure and label area.
- Protect containers and cylinders from physical damage.
Handling:
- All chemicals should be considered hazardous.
- Avoid direct physical contact.
- Use appropriate, approved safety equipment.
- Untrained individuals should not handle this chemical or its container.
- Handling should occur in a chemical fume hood.
Protection: Wear appropriate protective gloves, clothing, and goggles.
Respirators: Wear positive pressure self-contained breathing apparatus (SCBA).
At any detectable concentration: Any self-contained breathing apparatus with a full facepiece operated in a pressure-demand or other positive-pressure mode.
Any supplied-air respirator with a full facepiece operated in a pressure-demand or other positive-pressure mode or other positive-pressure mode with an auxiliary self-contained breathing apparatus operated in pressure-demand or other positive-pressure mode.
Escape – any air-purifying, full-facepiece respirator with a high-efficiency particulate filler.
Any appropriate escape-type, self-contained breathing apparatus.
Small spills and leaks:
- Do not touch damaged packages or spilled material.
- Cover liquid spill with sand, earth, or other noncombustible absorbent material.
- Cover the powder spill with a plastic sheet or tarp to minimize spreading.
- Contact the radiation safety officer.
5. FIREFIGHTING
The presence of radioactive material will not influence the fire control processes and should not influence the selection of techniques.
Small fires: Dry chemical, carbon dioxide, water spray, or regular foam.
Large fires: Water spray, fog (flooding amounts).
Firefighting and Other Immediately Dangerous to Life or Health Conditions
Use any self-contained breathing apparatus with a full facepiece respirator and a high-efficiency particulate filter.
Use any supplied-air respirator with a full facepiece operated in a pressure-demand or other positive-pressure mode or other positive-pressure modes with an auxiliary self-contained breathing apparatus operated in pressure-demand or other positive-pressure modes.
6. HEALTH
Exposure Effects
Ingestion, skin, and eyes: See inhalation.
Inhalation:
- There is minimal risk if the packaging remains intact.
- Package damage can result in a measurable release of radiation, but the risk is still low.
First Aid
Skin and eyes:
- Immediately flush with running water for at least 20 minutes.
- See ingestion.
Inhalation:
- Apply artificial respiration if the victim is not breathing.
- Administer oxygen if breathing is difficult.
- See Ingestion.
Ingestion:
- Medical problems take priority over radiological concerns.
- Use first aid treatment according to the nature of the injury.
- Do not delay the care and transport of a seriously injured person.
7. TRANSPORTATION
UN Number: 2909
Response Guide: 161
Hazard Class: 7
USCG CHRIS Code: RAD
Packing Group:
The classification of substances with multiple hazards must be determined following the criteria presented in the regulations mentioned above. Due to the various quantities and combinations of materials being shipped at one time, the information above must be determined based on the characteristics of the specific shipment.
Ammonium Uranyl Carbonate
IUPAC Name: uranium(VI)dioxide di-ammonium carbonate.
Other Names: uranyl ammonium carbonate.
Molar Mass: 522.199 g/mol
Melting Point: Decomposes between 165°C and 185°C
Solubility in water: Insoluble.
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references.
8. FURTHER INFORMATION
Copyright 2014 IBI Labs. License granted to make unlimited paper copies for internal use only.
IBI Labs requires that those who receive their materials comply with 29 CFR 1910.1200(h), which mandates that employers provide employees with effective information and training about hazardous chemicals in their workplace.
The contents of this document are believed to be accurate as of the date of revision and are provided in good faith. However, it is recommended that recipients use this information as supplementary and exercise caution and judgment regarding its accuracy and suitability. Please note that IBI Labs cannot be held responsible for any damage, direct or indirect, that occurs because of using the information provided in this Safety Data Sheet.
IBI Labs makes no warranties, expressed or implied, including warranties of merchantability and fitness for a particular purpose. This information is provided without warranty, and any use of the product that does not conform to this Safety Data Sheet, or that is used in combination with any other product or process, is the user’s responsibility.
Revision Date: 05/06/2024


